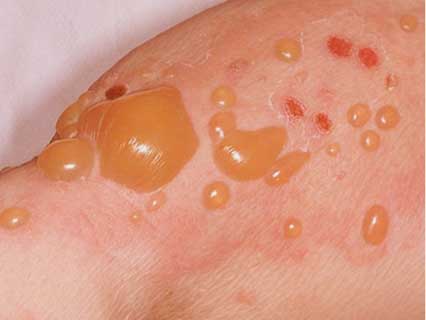
FDA Receives Reports Linking Type 2 Diabetes Drug With Serious Skin Diseases
Filed in: Type 2 Diabetes Drugs

Filed in: Type 2 Diabetes Drugs

Drug safety and availability - FDA drug safety communication: FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. (2015, August 28). Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm459579.htm
Class of diabetes drugs linked to painful blistering skin condition. (n.d.). Retrieved from https://staging.trulaw.com/diabetes/blistering-skin-conditions/
Merck and Pfizer announce two pivotal phase 3 studies for ertugliflozin, an investigational SGLT-2 inhibitor, met primary endpoints. (n.d.). Retrieved from http://www.pfizer.com/news/press-release/press-release-detail/merck_and_pfizer_announce_two_pivotal_phase_3_studies_for_ertugliflozin_an_investigational_sglt_2_inhibitor_met_primary_endpoints_showing_significant_a1c_reductions_in_patients_with_type_2_diabetes
Sloane, S. B. (n.d.). The DPP-4 inhibitor: lowers blood glucose by working on the gut. Retrieved from http://www.diabeticlivingonline.com/medication/oral/dpp-4-inhibitor-lowers-blood-glucose-working-gut