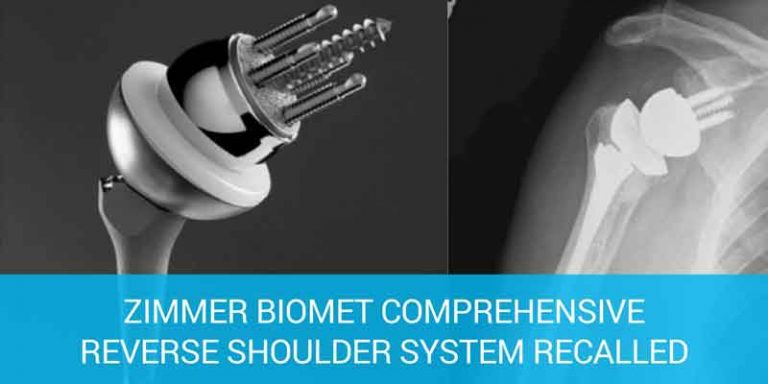
Zimmer Recalls Reverse Shoulder Device Due to Injury
Filed in: Reverse Shoulder Device Tags: reverse shoulder device

Filed in: Reverse Shoulder Device Tags: reverse shoulder device

Medical device recalls - Zimmer Biomet recalls comprehensive reverse shoulder due to a high fracture rate. (n.d.). Retrieved from https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm541862.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery
Reverse total shoulder replacement. (2017, March 01). Retrieved from http://orthoinfo.aaos.org/topic.cfm?topic=a00504
Zhou, H. S., Chung, J. S., Yi, P. H., Li, X., & Price, M. D. (2015, March). Management of complications after reverse shoulder arthroplasty. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4596185/