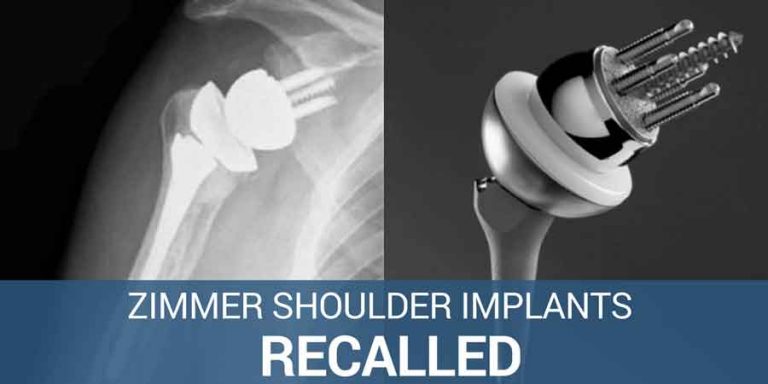
FDA Granted Fast-Track Approval to Now Recalled Zimmer Shoulder Implants
Filed in: Reverse Shoulder Device Tags: Shoulder Implants

Filed in: Reverse Shoulder Device Tags: Shoulder Implants

"510(k) Summary - K080642." Biomet Orthopedics, Inc. (n.d.): n. pag. U.S. Food and Drug Administration, 26 July 2012. Web. 18 May 2017. <https://www.accessdata.fda.gov/cdrh_docs/pdf8/K080642.pdf>.
Center for Devices and Radiological Health. "510(k) Clearances." U S Food and Drug Administration Home Page. Center for Devices and Radiological Health, 25 Jan. 2017. Web. 18 May 2017. <https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/510kClearances/>.
Center for Devices and Radiological Health. "Medical Device Recalls - Zimmer Biomet Recalls Comprehensive Reverse Shoulder Due to a High Fracture Rate." U S Food and Drug Administration Home Page. Center for Devices and Radiological Health, 16 Feb. 2017. Web. 18 May 2017. <https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm541862.htm>.
Gallo, Robert A., Seth C. Gamradt, Christopher J. Mattern, Frank A. Cordasco, Edward V. Craig, David M. Dines, and Russell F. Warren. "Instability after Reverse Total Shoulder Replacement." Elsevier Inc., June 2011. Web. 18 May 2017. <http://www.jshoulderelbow.org/article/S1058-2746(10)00385-X/fulltext>.