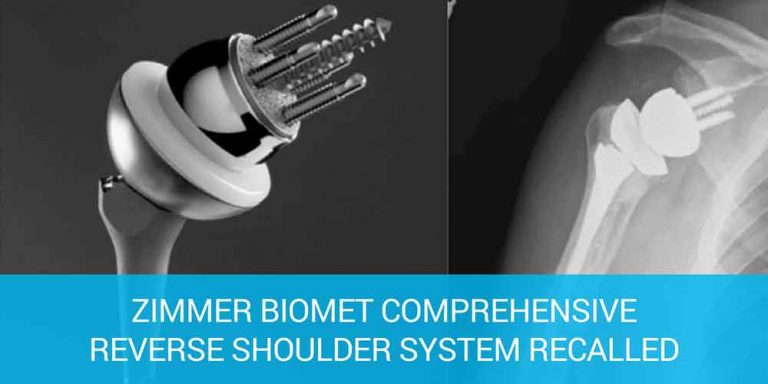
FDA Issues Class 1 Recall of Zimmer Shoulder Replacement Device
Filed in: Reverse Shoulder Device Tags: Zimmer Shoulder Replacement

Filed in: Reverse Shoulder Device Tags: Zimmer Shoulder Replacement

Medical device recalls - Zimmer Biomet Recalls Comprehensive Reverse Shoulder due to a high fracture rate. (2016, December 15). Retrieved from https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm541862.htm
Farshad, M., & Gerber, C. (2010, December). Reverse total shoulder arthroplasty—from the most to the least common complication. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2989053/
Ma, B. C., MD. (2010, December). Total shoulder replacement risks and complications. Retrieved from https://www.arthritis-health.com/surgery/shoulder-surgery/total-shoulder-replacement-risks-and-complications
Escapule, K. W. (2016). URGENT MEDICAL DEVICE RECALL-LOT SPECIFIC. International Law & World Order, 1-4. doi:10.1163/ilwo-ivc2c-3